Composition
- Each gastro-resistant tablets contains:
- Pantoprazole Sodium IP
eq. to Pantoprazole40 mg
Packing
- 10x10
(Alu-Alu)
MRP
- 59
Overview
Pantoprazole is a selective proton pump inhibitor drug (PPI) used for short-term treatment of erosion and ulceration of the esophagus caused by Gastroesophageal reflux disease. It acts by inhibiting the secretion of hydrochloric acid in the stomach by specific action on proton pumps of parietal cells.
WHAT IS PENTOM AND WHAT IT IS USED FOR?
Pantoprazole is used for relieving the symptoms and for the short team treatment of gastrointestinal diseases:- Duodenal ulcer
- Gastric ulcer
- Gastro-esophageal reflux disease
- Moderate and severe reflex oesophagitis
- Zollinger-Ellison syndrome and other hypersecretory conditions
- Prevention of gastro duodenal ulcers induced by non-selective non-steroidal anti-inflammatory drugs (NSAIDs).
Why is this medication prescribed?
Pantoprazole is used to treat certain conditions in which there is too much acid in the stomach. It is used to treat erosive esophagitis or "heartburn" caused by gastroesophageal reflux disease (GERD), a condition where the acid in the stomach washes back up into the esophagus. This medicine may also be used to treat Zollinger-Ellison syndrome, a condition where the stomach produces too much acid.Warnings
Before taking pantoprazole, tell your doctor or pharmacist if you are allergic to it; or to similar drugs (such as lansoprazole, omeprazole); or if you have any other allergies. This product may contain inactive ingredients, which can cause allergic reactions or other problems. Talk to your pharmacist for more details.
Symptomatic response does not preclude presence of gastric malignancy
Atrophic gastritis has been noted with long-term therapy
Bone Fracture: Long-term and multiple daily dose PPI therapy may be associated with an increased risk for osteoporosis-related fractures of the hip, wrist or spine.
Hypomagnesemia has been reported rarely with prolonged treatment with PPIs
Contraindications
Patients with a history of hypersensitivity to pantoprazole or to any constituents of the medication. It is also contraindicated in patients with cirrhosis of the liver and in cases of severe liver disease
Side Effects
Gastrointestinal: abdominal pain (3%), diarrhea (4%), flatulence (4%)
Neurologic: headache (5%)
Chest pain, headache, nausea, pain, anxiety
Dosage
Gastroesophageal reflux disease (treatment) Oral, 40 mg per day for up to eight weeks. An additional eight-week course may be considered in patients who have not healed after four to eight weeks of treatment.
Disclaimer:To be taken only after consulting with the doctor.
Storage
Store it at room temperature and away from excess heat and moisture. Throw away any medication that is outdated or no longer needed. Talk to your pharmacist about the proper disposal of your medication.
Pharmacology
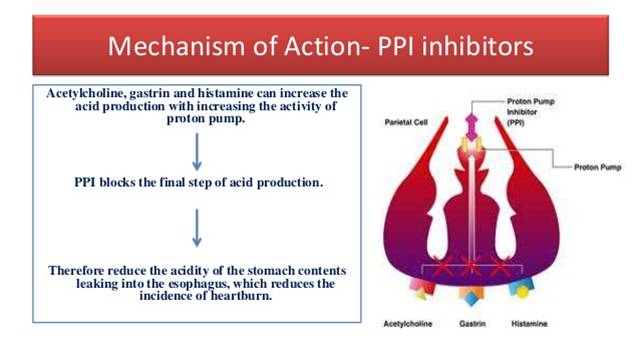
Mechanism of Action
Pantoprazole is a proton pump inhibitor (PPI) that suppresses the final step in gastric acid production by covalently binding to the (H+, K+)-ATPase enzyme system at the secretory surface of the gastric parietal cell. This effect leads to inhibition of both basal and stimulated gastric acid secretion, irrespective of the stimulus. The binding to the (H+, K+)-ATPase results in a duration of antisecretory effect that persists longer than 24 hours for all doses tested (20 mg to 120 mg).Pharmacokinetics
Absorption: Rapidly absorbed. However, absorption may be delayed up to 2 hours or more if pantoprazole is taken with food.Bioavailability (oral)- 77%.
Distribution: VolD - Following intravenous administration to extensive metabolizers: 0.17 L per kg (11 to 23.6 L).
Protein Binding: Very high (98%); primarily to albumin.
Half-Life: Elimination - Following oral or intravenous administration: 1 hour.
The half-life of pantoprazole is prolonged (7 to 9 hours) in patients with cirrhosis of the liver and in genetically determined slow metabolizers (3.5 to 10 hours).
Interactions
• Medicines such as ketoconazole, itraconazole and posaconazole (used to treat fungal infections) or erlotinib (used for certain types of cancer) because Pantoprazole tablets may stop these and other medicines from working properly.
• Warfarin and phenprocoumon, which affect the thickening, or thinning of the blood. You may need further checks.
• Atazanavir (used to treat HIV-infection).
Pregnancy and Breast-feeding:
There are no adequate data from the use of pantoprazole in pregnant women. Excretion into human milk has been reported. If you are pregnant, or think you may be pregnant, or if you are breast-feeding, you should use this medicine only if your doctor considers the benefit for you greater than the potential risk for your unborn child or baby.
For Patient
Take pantoprazole exactly as prescribed by your doctor. Follow all directions on your prescription label. Do not take this medicine in larger or smaller amounts or for longer than recommended.
Pantoprazole tablets can be taken with or without food. Pantoprazole oral granules should be taken 30 minutes before a meal.
Do not crush, chew, or break the tablet. Swallow it whole.
Take this medicine for the full prescribed length of time. Your symptoms may improve before the condition is fully treated. If you use pantoprazole for longer than 3 years, you could develop a vitamin B-12 deficiency. Talk to your doctor about how to manage this condition if you develop it. Call your doctor if your symptoms do not improve or if they get worse while you are taking this medicine. If you use pantoprazole for longer than 3 years, you could develop a vitamin B-12 deficiency. Talk to your doctor about how to manage this condition if you develop it. This medicine can cause you to have a false positive drug screening test. If you provide a urine sample for drug screening, tell the laboratory staff that you are taking pantoprazole.
Chemistry
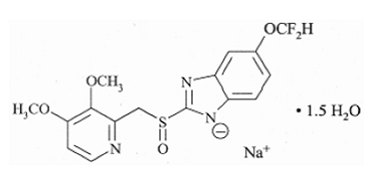
Pantoprazole sodium) Delayed-Release Tablets is a substituted benzimidazole, sodium 5-(difluoromethoxy)-2-[[(3,4-dimethoxy-2-pyridinyl)methyl] sulfinyl]1H-benzimidazole
sesquihydrate, a compound that inhibits gastric acid secretion. Its empirical formula is C16H14F2N3NaO4S x 1.5 H2O, with a molecular weight of 432.4.
Pantoprazole sodium sesquihydrate is a white to off-white crystalline powder and is racemic. Pantoprazole has weakly basic and acidic properties. Pantoprazole sodium sesquihydrate is freely soluble in water, very slightly soluble in phosphate buffer at pH 7.4, and practically insoluble in n-hexane. The stability of the compound in aqueous solution is pH-dependent. The rate of degradation increases with decreasing pH. At ambient temperature, the degradation half-life is approximately 2.8 hours at pH 5 and approximately 220 hours at pH 7.8.
Clinical Data
| AU: B3 |
Legal Status | Rx Prescription |
Routes | Oral |