Composition
- Each Film coated Tablet Contains:
- Ondansetron Hydrochloride IP
4 mg
Packing
- 10x10
(Alu-Alu)
MRP
- 42
Overview
Ondansetron blocks the actions of chemicals in the body that can trigger nausea and vomiting. Ondansetron is used to prevent nausea and vomiting that may be caused by surgery or by medicine to treat cancer (chemotherapy or radiation). Ondansetron is not for preventing nausea or vomiting that is caused by factors other than cancer treatment or surgery.
WHAT IS ATOMSET AND WHAT IT IS USED FOR?
ATMOSET tablets contain a medicine called ondansetron. This belongs to a group of medicines called anti-emetics. ATMOSET tablets are used for: preventing nausea and vomiting caused by chemotherapy or radiotherapy for cancer preventing nausea and vomiting after surgery. Ask your doctor, nurse or pharmacist if you would like any further explanation about these uses. ATMOSET tablets should start to work within one or two hours of taking a dose.Why is this medication prescribed?
This medication is used alone or with other medications to prevent nausea and vomiting caused by cancer drug treatment (chemotherapy) and radiation therapy. It is also used to prevent and treat nausea and vomiting after surgery. It works by blocking one of the body's natural substances (serotonin) that causes vomiting. Ondansetron belongs to a class of medications called 5-HT3 blockers.Warnings
Do not take ATMOSET:
Check with your doctor or pharmacist before taking ATMOSET tablets if:
you have ever had heart problems
you are allergic to medicines similar to ondansetron, such as granisetron
you have an uneven heart beat (arrhythmias)
you have liver problems
you have a blockage in your gut or suffer from severe constipation
you have problems with the levels of salts in your blood, such as potassium, sodium and magnesium.
Contraindications
The concomitant use of apomorphine with Ondansetron is contraindicated based on reports of profound hypotension and loss of consciousness when apomorphine was administered with Ondansetron. Ondansetron tablets and Ondansetron orally disintegrating tablets are contraindicated for patients known to have hypersensitivity to the drug.
Dosage
Disclaimer:To be taken only after consulting with the doctor.Storage
Keep ATMOSET Tablets in a cool, dry place where it stays below 30°C, and away from bright sunlight.
Pharmacology
Mechanism of Action
- Selective 5-HT3 (serotonin) receptor antagonists
- Serotonin receptors of the 5-HT3 type are present both peripherally on vagal nerve terminals and centrally in the chemoreceptor trigger zone of the area postrema
- It is not certain whether ondansetron's antiemetic action in chemotherapy-induced emesis is mediated centrally, peripherally, or in both sites Ondansetron is a selective 5-HT3 receptor antagonist. While ondansetron's mechanism of action has not been fully characterized, it is not a dopamine-receptor antagonist. Serotonin receptors of the 5-HT3 type are present both peripherally on vagal nerve terminals and centrally in the chemoreceptor trigger zone of the area postrema. It is not certain whether ondansetron's antiemetic action in chemotherapy-induced nausea and vomiting is mediated centrally, peripherally, or in both sites. However, cytotoxic chemotherapy appears to be associated with release of serotonin from the enterochromaffin cells of the small intestine.
Pharmacokinetics
Ondansetron is well absorbed from the gastrointestinal tract and undergoes some first-pass metabolism. Mean bioavailability in healthy subjects, following administration of a single 8-mg tablet, is approximately 56%.Ondansetron systemic exposure does not increase proportionately to dose. AUC from a 16-mg tablet was 24% greater than predicted from an 8-mg tablet dose. This may reflect some reduction of first-pass metabolism at higher oral doses. Bioavailability is also slightly enhanced by the presence of food but unaffected by antacids.
Ondansetron is extensively metabolized in humans, with approximately 5% of a radiolabeled dose recovered as the parent compound from the urine. The primary metabolic pathway is hydroxylation on the indole ring followed by subsequent glucuronide or sulfate conjugation. Although some nonconjugated metabolites have pharmacologic activity, these are not found in plasma at concentrations likely to significantly contribute to the biological activity of ondansetron. In vitro metabolism studies have shown that ondansetron is a substrate for human hepatic cytochrome P-450 enzymes, including CYP1A2, CYP2D6, and CYP3A4. In terms of overall ondansetron turnover, CYP3A4 played the predominant role. Because of the multiplicity of metabolic enzymes capable of metabolizing ondansetron, it is likely that inhibition or loss of one enzyme (e.g., CYP2D6 genetic deficiency) will be compensated by others and may result in little change in overall rates of ondansetron elimination. Ondansetron elimination may be affected by cytochrome P-450 inducers.
Interactions
Ondansetron does not itself appear to induce or inhibit the cytochrome P-450 drug-metabolizing enzyme system of the liver. Because ondansetron is metabolized by hepatic cytochrome P-450 drug-metabolizing enzymes (CYP3A4, CYP2D6, CYP1A2), inducers or inhibitors of these enzymes may change the clearance and, hence, the half-life of ondansetron. On the basis of available data, no dosage adjustment is recommended for patients on these drugs.
Phenytoin, Carbamazepine, and Rifampicin: In patients treated with potent inducers of CYP3A4 (i.e., phenytoin, carbamazepine, and rifampicin), the clearance of ondansetron was significantly increased and ondansetron blood concentrations were decreased. However, on the basis of available data, no dosage adjustment for ondansetron is recommended for patients on these drugs.
Tramadol: Although no pharmacokinetic drug interaction between ondansetron and tramadol has been observed, data from 2 small studies indicate that ondansetron may be associated with an increase in patient controlled administration of tramadol.
Chemotherapy: Tumor response to chemotherapy in the P-388 mouse leukemia model is not affected by ondansetron. In humans, carmustine, etoposide, and cisplatin do not affect the pharmacokinetics of ondansetron.
For Patients
Information for Patients
When you must not use them:
- Do not use ATMOSET Tablets after the expiry or "use by" date (EXP) printed on the pack. If you take it after the expiry date it may not work as well.
- Do not use ATMOSET Tablets if the packaging is torn or shows signs of tampering or if the tablets look damaged or discoloured.
- Do not use ATMOSET if you are taking apomorphine (used to treat Parkinson's disease) .
- Do not take ATMOSET Tablets if you have ever had an allergic reaction to ondansetron or any of the other ingredients in ATMOSET, which are listed at the end of this leaflet. Before you take Zofran Tablets .
- Tell your doctor if you are pregnant, trying to become pregnant or breastfeeding. Your doctor will discuss the risks and benefits with you.
- If you have had to stop taking another medicine for your nausea or vomiting, tell your doctor. If you are allergic to any foods, dyes, preservatives or any other medicine, tell your doctor.
- If you have or used to have liver problems, tell your doctor.
- Tell your doctor if you are constipated.
- Tell your doctor if you have, or used to have, heart problems.
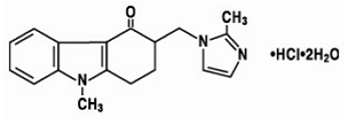
Chemistry
The active ingredient in ATMOSET Tablets is ondansetron hydrochloride (HCl) as the dihydrate, the racemic form of ondansetron and a selective blocking agent of the serotonin 5-HT3 receptor type. Chemically it is (±) 1, 2, 3, 9-tetrahydro-9-methyl-3- [(2-methyl-1H-imidazol-1-yl) methyl]-4H-carbazol-4-one, monohydrochloride, dihydrate. It has the following structural formula:
The empirical formula is C18H19N3O•HCl•2H2O, representing a molecular weight of 365.9.
Ondansetron HCl dihydrate is a white to off-white powder that is soluble in water and normal saline.
Clinical Data
Pregnancy Category |
AU: B1 US: B |
Legal Status | AU: Prescription Only (S4) CA: Rx-only UK: POM US: Rx-only |
Routes | Oral |