Composition
- Each Uncoated Tablet Contains:
- Cinnarizine IP
20 mg
- Domperidone Maleate IP
15 mg
Packing
- 10x10
(Alu-Alu)
MRP
- 45
Overview
ATMOZIN-D is a complete antiemetic and antivertigo drug. It is a combination of Domperidone & Cinnarizine that takes care of the symptoms of nausea and vomiting as well as the root cause of motion sickness respectively.
WHAT IS ATMOZIN-D AND WHAT IT IS USED FOR?
ATMOZIN-D is antiemetic and antivertigo drug. Domperidone is a selective peripheral dopamine antagonist at the D2 receptor. It acts on D2 receptors at the CETZ and stomach but it does not readily cross blood brain barrier (BBB). It is structurally related to butyrophenones with antiemetic and gastroprokinetic properties. Domperidone Maleate increases the lower oesophageal sphincter (LES) pressure, promotes oesophageal peristalsis increases gastric emptying time and frequency, amplitude and durations of duodenal contractions. Cinnarizine has a centrally acting antihistamine (H1) effect and a calcium channel antagonist. Cinnarizine depresses labyrinth and acts as a peripheral vasodilator. Cinnarizine also reduces blood viscosity but does not have any effect on blood pressure and heart rate.
INDICATIONS
• Cinnarizine is predominantly used to treat nausea and vomiting associated with motion sickness, vertigo, Ménière's disease, or Cogan's syndrome. In fact, it is one of only a select few drugs that has shown a beneficial effect in the chronic treatment of the vertigo and tinnitus, associated with Meniere's disease.
- Vestibular disorders
- Prophylaxis and control of Motion sickness
- Nausea and vomiting of varied aetiology.
Why is this medication prescribed?
ATMOZIN is a combination of Cinnarizine 20mg and Domperidone 15mg. This combination of Cinnarizine and Domperidone would be ideal for the management of vestibular disorders prophylaxis and control of motion sickness and nausea & vomiting of varies aetiologies.
Warnings
This medicine may lead to drowsiness and impaired concentration, which may be aggravated by simultaneous intake of alcohol or other central nervous system depressants..
Patients should not operate hazardous machinery or drive motor vehicles or perform potentially hazardous tasks where loss of concentration may lead to accidents.
Contraindications
Cinnarizine + Domperidone and Pregnancy
Caution when used during pregnancyCinnarizine + Domperidone and Lactation
Caution when used during lactationCinnarizine + Domperidone and Other Contraindications
Hypersensitivity. Parkinson's disease. GI haemorrhage, obstruction and perforation, patients with prolactin releasing pituitary hormone or chronic admin or for prophylaxis of postoperative nausea and vomiting. Neonates, children.Side Effects
Cinnarizine is well tolerated; commonly reported side effects were sedation.gastric irritation, rashes and drowsiness.
Central effects such as extra pyramidal reactions or drowsiness may be lower.
Dosage
Oral:This is Preferred Dosage:
Motion sickness
Adult: Each tablet contains cinnarizine 20 mg and domperidone 15 mg: 1 tablet taken 1-2 hours before travel and 1 tablet every 6 hours during journey if necessary.
Disclaimer:To be taken only after consulting with the doctor.
Storage
Keep all medicines out of the reach and sight of children. Store in a cool,dry place, away from direct heat and light.
Pharmacology
Cinnarizine has Ca channel blocking activity selective for arterial smooth muscles. It has some antihistamine activity. Cinnarizine acts as a labyrinthine sedative. It also improves microcirculation by reducing ischaemia-induced blood viscosity. Domperidone is a peripheral dopamine-receptor blocker. It increases both the oesophageal peristalsis and the lower oesophageal sphincter pressure. It also increases gastric motility and peristalsis, and enhances gastroduodenal coordination, thus facilitating gastric emptying and decreasing small bowel transit time.
Pharmacodynamics
Cinnarizine has antihistaminic, sedative and calcium channel blocking activity. Cinnarizine by virtue of its calcium channel blocking activity relaxes vascular smooth muscles. It has been found to modulate calcium fluxes and attenuates vasoconstrictor action probably by its action on calcium movement. It improves blood flow to labyrinth and brainstem, also acts as labyrinth sedative.Domperidone possesses both prokinetic and antiemetic properties. Domperidone is a dopaminergic and it is postulated that it causes antiemetic action by blocking dopamine receptors, also it increases gastric motility. Domperidone crosses the blood brain barrier to only a limited extent, and it causes extra pyramidal side effects very rarely.
Pharmacokinetics
Cinnarizine is absorbed from the gastro intestinal tract, peak plasma concentrations occurring 2 to 4 hrs after oral administration. It undergoes metabolism and has a half life of 3 to 6 hrs. Cinnirazine is excreted mainly in the feces as unchanged drug and in urine mainly as its metabolites.Domperidone bioavailability is increased when it is administered after food compared to before food. Peak plasma is reached within 30 minutes following the administration of dose, 90% is bound to plasma protein and has a half life is 7.5 hrs. About 30% of the dose is excreted in urine within 24 hrs almost entirely as metabolites and the remaining as feces over several days.
Interactions
- CNS depressant effect of cinnarizine is enhanced with alcohol.
- Domperidone reduces absorption of oral digoxin. It increases absorption of aspirin, paracetamol and oral diazepam.
- Enhances CNS depression by phenothiazine.
- Antimuscarinic agents and opioids antagonise GI effects. Prolongs suxamethonium-induced neuromuscular blockade.
For Patient
Some medicines are not suitable for people with certain conditions, and sometimes a medicine may only be used if extra care is taken. For these reasons, before you start taking cinnarizine it is important that you should know:- If you are pregnant, trying for a baby or breast-feeding.
- If you have liver or kidney problems.
- If you have glaucoma (increased pressure in your eye).
- If you have epilepsy.
- If you have Parkinson's disease.
- If you have prostate problems, or have been experiencing difficulty passing urine.
- If you know you have a blockage in your small intestines.
- If you have porphyria (a rare blood disorder).
- If you are taking other medicines, including those available to buy without a prescription, herbal and complementary medicines.
- If you have ever had an allergic reaction to this or to any other medicine.
HOW TO TAKE CINNARIZINE
- Before you start this treatment, read the manufacturer's printed information leaflet from inside your pack. The leaflet will give you more information about the specific brand of cinnarizine you have been given, and a full list of possible side-effects from taking it.
- Take cinnarizine exactly as your doctor or pharmacist has told you to.
- If you are taking cinnarizine to relieve travel sickness, take the first dose two hours before you are due to travel. If you are going on a long journey, you can then take further doses every eight hours if needed. If you are giving cinnarizine to your child, check the label carefully to make sure you are giving the correct doses for the age of your child. Make sure you leave eight hours between each dose.
- If you are able, take cinnarizine with a snack or just after eating a light meal. This will help to prevent any stomach upset.
Chemistry
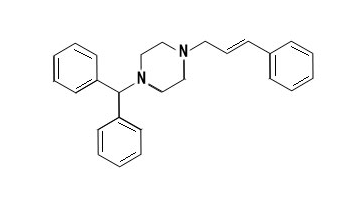
CinnarizineCinnarizine where in the former is classified under Antihistamines and the later classified under Calcium channel blockers are derivatives of piperazine. Piperazines are a broad class of chemical compounds, many with important pharmacological properties. Drugs of different pharmacological actions can be designed by mono or di substitution of the two nitrogen atoms present on the piperazine structure. Cinnarizine is di substituted derivative of piperazine, which is grouped under the class diphenyl methyl piperazines of piperazine.
Structure of Cinnarizine:
IUPAC Name:
1-[di(phenyl)methyl]-4-(3'-phenylprop-2'-enyl)piperazine.
Chemical Formula: C26H28N2
Synthesis:
Reaction of benzydryl chloride with N-acylpiperazine gives monosubstituted piperazine. Acylation of the monosubstituted piperazine, with cinnamoyl chloride gives the corresponding amide. Reduction of the carbonyl by means of lithium aluminums hydride affords Cinnarizine.
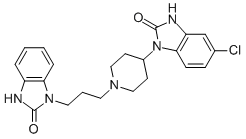
Drug Substance:
Proper Name: Domperidone Maleate
ChemicalName:5-chloro-1-[1-[3-(2,3-dihydro-2-oxo-1H-benzimidazol-1-yl)propyl]-4-piperidinyl]-1,3-dihydro-2H-benzimidazol-2-one(Z)-2-butenedioate (1:1)
Molecular Formula: C22H24N5O2Cl•C4H4 04
Molecular Weight: 541.99 g/mol
Description: Domperidone maleate is a white to off white crystalline powder, soluble in N,Ndimethylformamide, and insoluble in water with a melting range of 223-231°C.
Clinical Data of Cinnarizine
Pregnancy Category | US: C |
Legal Status | --- |
Routes | Oral |
Clinical Data of Domperidone
Pregnancy Category | Not classified (US) |
Legal Status | Not approved for use or sale: US; prescription medicine (POM): India, Australia, Canada, Israel, Belgium; Over the Counter (OTC): UK (Prescription Only Medicine as of 4th September 2014), Egypt, Ireland, Italy, Japan, Netherlands, South Africa, Switzerland, China, Russia, Slovakia, Thailand, Malta, South Korea, and Romania[1] |
Routes | Oral |