Composition
- Each Film Coated Tablet Contains:
- Fexofenadine Hydrochloride IP
120 mg
- Montelukast Sodium IP
10 mg
Packing
- 1x10, 10x1x10
(Alu Alu)
MRP
- 119
Indications
ATMOKAST-FX is indicated for relief of symptoms of allergic rhinitis [seasonal or perennial], prophylaxis in seasonal allergic rhinitis and in treatment of asthma. It is indicated in the treatment of asthma as add-on therapy in those patients with mild to moderate persistent asthma who are inadequately controlled on inhaled corticosteroids.
Warnings
Patients with history of or ongoing cardiovascular disease should be warned that, antihistamines as a drug class (Fexofenadine) have been associated with the adverse events, tachycardia and palpitation.
Patients should be advised never to use oral Montelukast to treat acute asthma attacks and to keep their usual appropriate rescue medication for this purpose readily available. If an acute attack occurs, a short-acting inhaled beta-agonist should be used. Patients should seek their doctors' advice as soon as possible if they need more inhalations of short-acting beta-agonists than usual. Montelukast should not be substituted for inhaled or oral corticosteroids.Contraindications
Hypersensitivity to the active substance or to any of the Excipients
Hepatic Impairment: As Montelukast is mainly excreted through bile, caution is to be exercised while prescribing this combination in patients with impaired hepatic function.Side Effects
Vomiting, dizziness, fatigue, fever; rash; abdominal pain, gastroenteritis; weakness; cough, nasal congestion, aggression, diarrhoea, drowsiness, hypersensitivity, nausea.
Dosage
This is Preferred Dosage:
The recommended dose is one tablet daily or as directed by physician.
Disclaimer:To be taken only after consulting with the doctor.
Storage
Store in cool, dry & dark place.
Pharmacology
Mechanism of Action
Fexofenadine: Like other H1-blockers, Fexofenadine competes with free histamine for binding at H1-receptors in the GI tract, large blood vessels, and bronchial smooth muscle. This block the action of endogenous histamine, which subsequently leads to temporary relief of the negative symptoms (eg. nasal congestion, watery eyes) brought on by histamine. Fexofenadine exhibits no anticholinergic, antidopaminergic, alpha1-adrenergic or beta-adrenergic-receptor blocking effects.Montelukast selectively antagonizes leukotriene D4 (LTD4) at the cysteinyl leukotriene receptor, CysLT1, in the human airway. Montelukast inhibits the actions of LTD4 at the CysLT1 receptor, preventing airway edema, smooth muscle contraction, and enhanced secretion of thick, viscous mucus.
Pharmacodynamics
Fexofenadine is a second-generation, long lasting H1-receptor antagonist (antihistamine) which has a selective and peripheral H1-antagonist action. Histamine is a chemical that causes many of the signs that are part of allergic reactions, such as the swelling of tissues. Histamine is released from histamine-storing cells (mast cells) and attaches to other cells that have receptors for histamine. The attachment of the histamine to the receptors causes the cell to be "activated," releasing other chemicals which produce the effects that we associate with allergy. Fexofenadine blocks one type of receptor for histamine (the H1 receptor) and thus prevents activation of cells by histamine. Unlike most other antihistamines, Fexofenadine does not enter the brain from the blood and, therefore, does not cause drowsiness.Fexofenadine lacks the cardiotoxic potential of terfenadine, since it does not block the potassium channel involved in repolarization of cardiac cells.
Montelukast, like zafirlukast, is a leukotriene receptor antagonist used as an alternative to anti-inflammatory medications in the management and chronic treatment of asthma and exercise-induced bronchospasm (EIB). Unlike zafirlukast, montelukast does not inhibit CYP2C9 or CYP3A4 and is, therefore, not expected to affect the hepatic clearance of drugs metabolized by these enzymes.
Pharmacokinetics
FexofenadineAbsorption: Fexofenadine hydrochloride is rapidly absorbed into the body following oral administration, with Tmax occurring at approximately 1-3 hours post dose. The mean Cmax value was approximately 427 ng/ml following the administration of a 120 mg dose once daily.(33%).
Distribution: Fexofenadine is 60-70% plasma protein bound.
Metabolism : Approximately 5% of the total dose is metabolized, by cytochrome P450 3A4 and by intestinal microflora.
Half Life: 14.4 hours
Excretion: The major route of elimination is believed to be via biliary excretion while up to 10% of ingested dose is excreted unchanged through the urine.
Montelukast
Absorption: Montelukast is rapidly absorbed following oral administration. The mean oral bioavailability is 64%.
Distribution: Montelukast is more than 99% bound to plasma proteins. The steady-state volume of distribution of montelukast averages 8-11 liters.
Half Life: 2.7-5.5 hours.
Metabolism: Hepatic (CYP3A4 and CYP2C9-mediated).
Elimination: Montelukast and its metabolites are excreted almost exclusively via the bile.
Interactions
Fexofenadine
• Cisapride: increased risk of cardiotoxicity and arrhythmias.
• The therapeutic effects of the central acetylcholinesterase inhibitor, Tacrine, and/or the anticholinergic, Fexofenadine, may be reduced due to antagonism. The interaction may be beneficial when the anticholinergic action is a side effect. Monitor for decreased efficacy of both agents.
• Trimethobenzamide and Fexofenadine, two anticholinergics, may cause additive anticholinergic effects and enhance their adverse/toxic effects. Monitor for enhanced anticholinergic effects.
• Triprolidine and Fexofenadine, two anticholinergics, may cause additive anticholinergic effects and enhance their adverse/toxic effects. Additive CNS depressant effects may also occur. Monitor for enhanced anticholinergic and CNS depressant effects.
• Trospium and Fexofenadine, two anticholinergics, may cause additive anticholinergic effects and enhanced adverse/toxic effects. Monitor for enhanced anticholinergic effects.
Montelukast
• Tolbutamide, a strong CYP2C9 inhibitor, may decrease the metabolism and clearance of Montelukast.
For Patient
What should I know before taking the medicine?Some medicines are not suitable for people with certain conditions, and sometimes a medicine may only be used if extra care is taken. For these reasons, before you (or your child) start taking fexofenadine it is important that your doctor knows:
- If you are pregnant, trying for a baby or breast-feeding.
- If you have ever had an allergic reaction to another antihistamine, or to any other medicine.
- Before you start taking the tablets, read the manufacturer's printed information leaflet from inside the pack. The manufacturer's leaflet will give you more information about Montelukast & Fexofenadine and a full list of the side-effects which you may experience from taking it.
- If you do forget to take a dose, don't worry, but do remember to take your next dose when it is due. Do not take two doses together to make up for a forgotten dose.
- Some antacids can reduce the amount of fexofenadine your body absorbs. Because of this, it is recommended that you do not take indigestion remedies during the two hours before or during the two hours after you take a fexofenadine tablet.
Chemistry
Fexofenadine is a third generation Antihistamine used in the treatment of allergy symptoms, such as hay fever or other upper respiratory allergies: runny nose, sneezing, itching of the nose or throat and itchy, watery eyes, nasal congestion and Urticaria.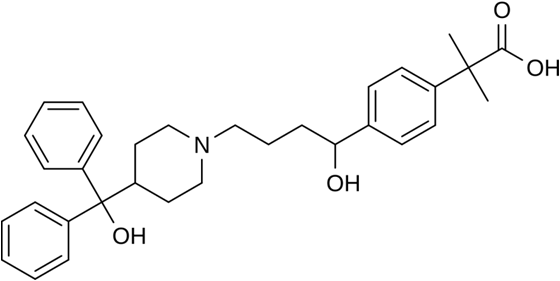
4-(1-Hydroxy-4-(4-(hydroxydiphenylmethyl)-1-piperidinyl)butyl)-alpha,alpha-dimethylbenzeneacetic acid
Montelukast is a leukotriene receptor antagonist (LTRA) used for the maintenance treatment of asthma and to relieve symptoms of seasonal allergies. Montelukast is a CysLT1 antagonist; it blocks the action of leukotriene D4 (and secondary ligands LTC4 and LTE4) on the cysteinyl leukotriene receptor CysLT1 in the lungs and bronchial tubes by binding to it. This reduces the bronchoconstrictionotherwise caused by the leukotriene and results in less inflammation.
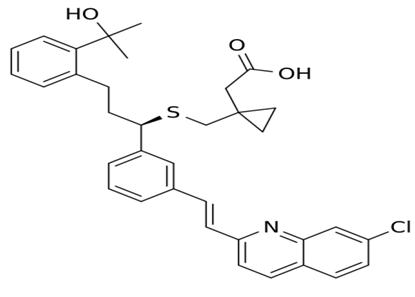
(R,E)-2-(1-((1-(3-(2-(7-chloroquinolin-2-yl)vinyl)phenyl)-3-(2-(2-hydroxypropan-2-yl)phenyl)propylthio)methyl)cyclopropyl)acetic acid
Clinical Data
Clinical Data of Fexofenadine
Pregnancy Category | AU: B2 US: C |
Legal status | AU: Unscheduled CA: OTC UK: POM US: OTC |
Routes | Oral |
Chemical Data
Formula | C32H39NO4 |
Molecular Mass | 501.68 |
Clinical Data of Montelukast
Pregnancy Category | AU: B1 US: B |
Legal status | AU: Prescription Only (S4) CA: Rx-only UK: POM US:Rx-only |
Routes | Oral |
Chemical Data
Formula | C35H36ClNO3S |
Molecular Mass | 586.184 g/mol |